Novel Coronavirus 2019-nCoV nucleic acid detection kit realizes the rapid screening of the addition of novel Coronavirus samples within 1 minute, without nucleic acid extraction, meeting the needs of high-throughput detection, and can provide a fast, simple, accurate and high-throughput nucleic acid detection solution for epidemic prevention and control.
Novel Coronavirus 2019-nCoV Nucleic Acid Detection Kit (Fluorescent PCR)
Sample type: throat swab, sputum
[Medical Device Registration Certificate No. / Product Technical Requirement No.] National Instrument Registration Permit 20203400644
This Flash DetectTM SARS-CoV-2 Detection Kit is used for in vitro qualitative detection of novel coronavirus(2019-nCoV) ORF1Ab and N genes in throat swabs
and sputum samples of suspected cases of pneumonia infected by novel coronavirus(2019-nCoV), suspected clusters of patients, and other patients who need to be diagnosed or differential diagnosed by novel coronavirus infection.
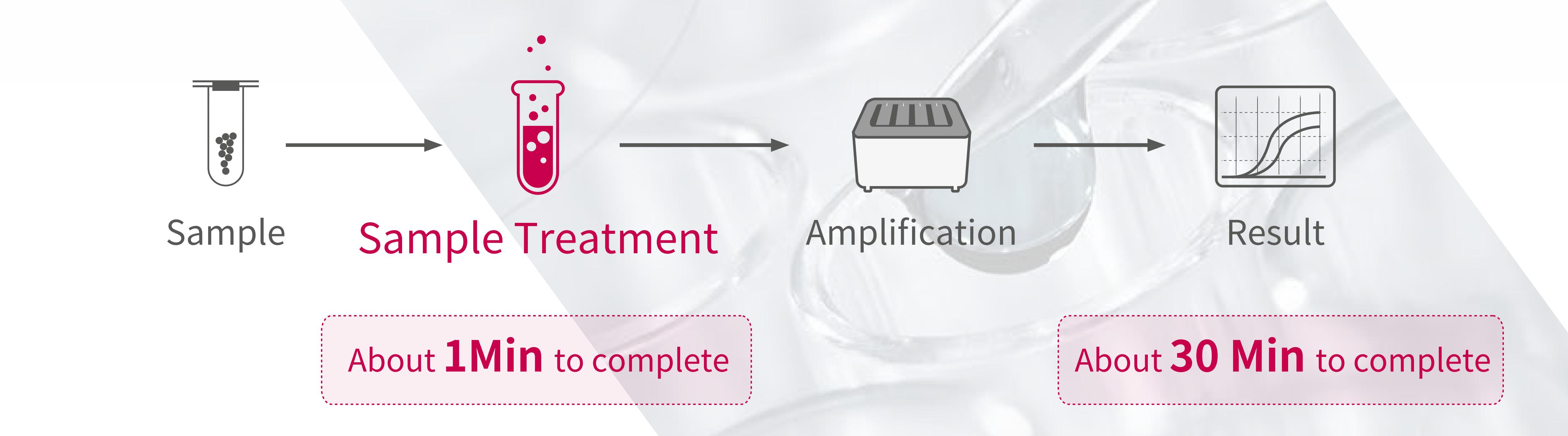
Novel Coronavirus 2019-nCoV nucleic acid assay is a real-time assay based on nucleic acid free extraction PCR (fluorescent probe method).
• Provide reliable evidence for medical decisions, effectively help identify suspected cases, especially hidden infections, and avoid the spread of the wider epidemic.
• It is easy for medical staff to achieve targeted treatment quickly.
Providing rapid patient testing results can:
• Helps patients manage their treatment.
• Helps in rapid triage of patients and prevents cross-infection.
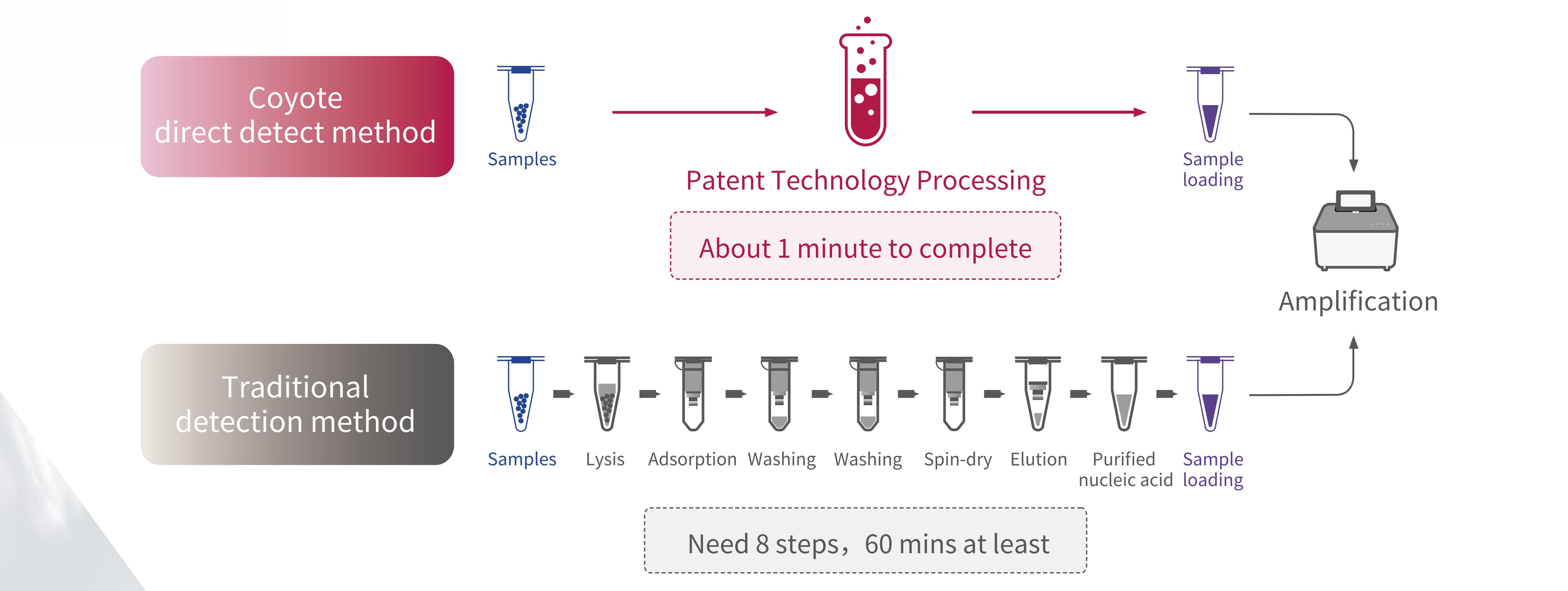
• Easy to use without nucleic acid extraction, free laboratory staff
• Safely reduce the risk of contact between experimenters and samples
• Add samples quickly in 1 minute and get results in 30 minutes
• Scene central laboratory, laboratory, medical test center, disease control center
• A suspected case of pneumonia from Novel Coronavirus
• Suspected cluster cases
• Other persons requiring a diagnosis of Novel Coronavirus infection or differential diagnosis
7. Applicable instruments
|
This kit is suitable for ABI 7500, Bio-Rad CFX96, and Coyote ®Flash20.
Sue machinery wide review (article) : 210712-02561 | taboo content or considerations can be found in the manual.
|
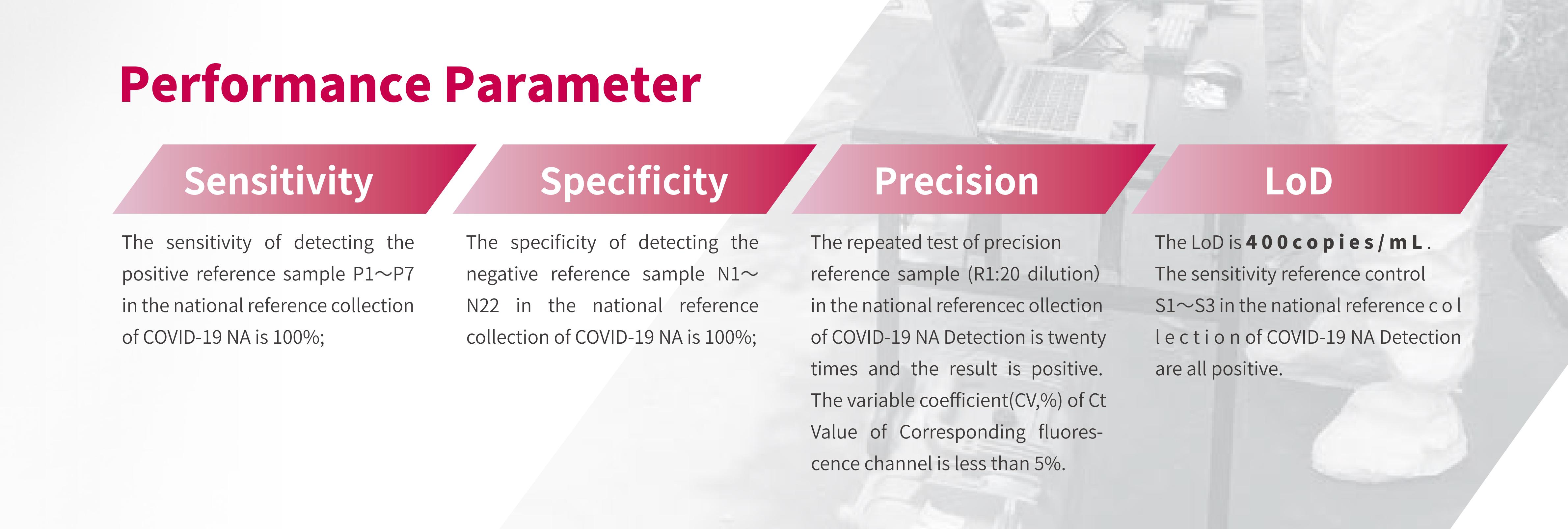
8. Evaluation Results of Trusted Authority
|

|
11.Direct Detect Technology VS Traditional Technology
|

























 Request for Quotation
Request for Quotation

